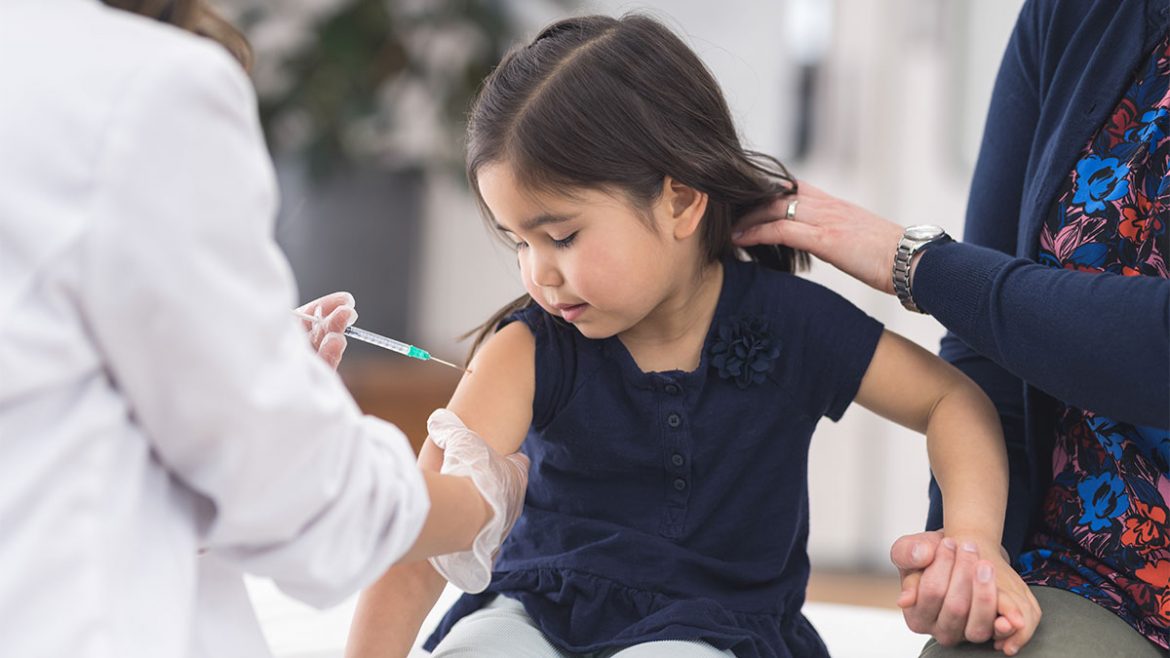
On Monday, Pfizer and BioNTech announced that their coronavirus vaccine was safe and produced a strong immune response in children aged five to eleven, and that they would seek regulatory approval soon.
The vaccine would be administered at a lower dosage than for people over 12, they said.
“In participants five to 11 years of age, the vaccine was safe, well-tolerated and showed robust neutralising antibody responses,” US giant Pfizer and its German partner said in a joint statement.
They plan to submit their data to regulatory bodies in the European Union, the United States and around the world “as soon as possible”.
The trial results are the first of their kind for children under 12, with a Moderna trial for six-11-year-olds still ongoing.
Both the Pfizer and Moderna jabs are already being administered to adolescents over 12 and adults in countries around the globe.
“We are eager to extend the protection afforded by the vaccine to this younger population,” said Pfizer CEO Albert Bourla, noting that “since July, paediatric cases of COVID-19 have risen by about 240 per cent in the US”.
Kids in the 5-11 age trial group received a two-dose regimen of 10 microgrammes in the trial, compared with 30 microgrammes for older age groups, the companies said. The shots were given 21 days apart.